The "Swiss Army Knife" Longevity Target NLRP3 Strikes Again
This post is not an investment advise or any form of endorsement of any of the drugs. To my knowledge, all of the NLRP3 drugs are investigational and none is approved for clinical use.
In a previous post on Forever.ai titled “Beyond GLP-1: Is NLRP3 the Next Trillion Dollar Target?”, I argued that the NLRP3 inflammasome was the next trillion-dollar target, a “master switch” for the chronic, low-grade inflammation—or “inflammaging”—that drives the majority of age-related diseases. At least two longevity-focused companies, Insilico and BioAge prioritized it for their pipelines going after best-in-class brain-penetrant NLRP3 inhibitors. Insilico chose Parkinson’s at the primary clinical pathway and BioAge chose obesity. What was then a strategic AI-derived forecast is now rapidly becoming a clinical and commercial reality. The catalyst for this update is a surge of fresh Phase 2 clinical results that have underscored the significant potential of NLRP3 inhibition. Recent data from Ventyx Biosciences was very promising supporting the hypothesis that NLRP3 inhibitors may be the “third pillar” of preventative cardiometabolic medicine alongside statins and GLP-1 agonists. At the same time, a powerful convergence of clinical signals in Parkinson’s disease is providing the first real hope for a therapy that can potentially modify the course of neurodegeneration.
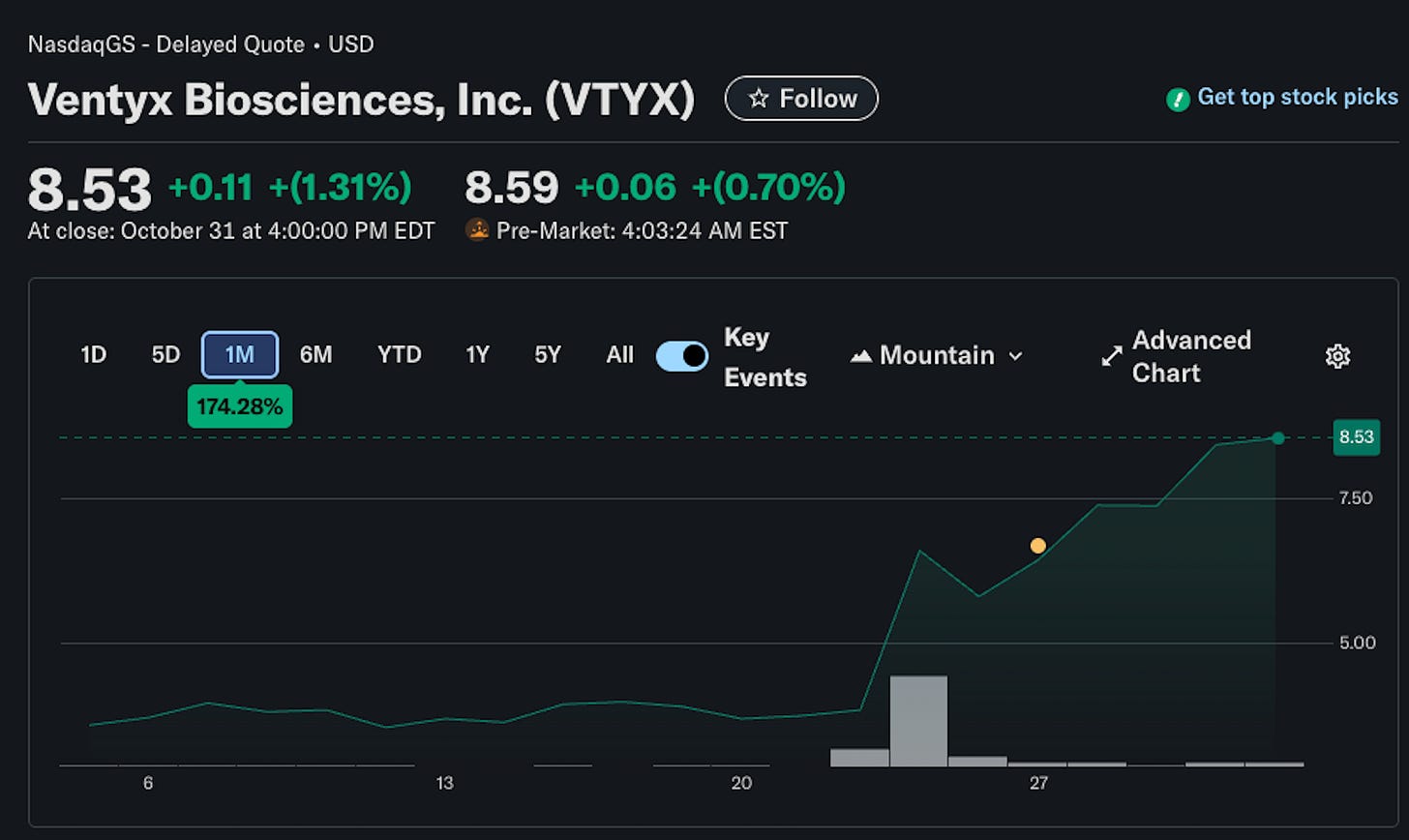
But to make it in the NLRP3 world, many stars need to align. You need to have very safe molecule, strategic commitment and funding, great scientists, and perfect clinical trial design. Just this week, we saw Ventus discontinue their NLRP3 program in PD. Even before that, most NLRP3 developers did not consider them to be a serious competitor in this space. But Ventyx stock gained considerably since the news were announced - now there are even fewer competitors in PD.
This report will dissect these new results, exploring what they mean for inflammation-centric therapies and surveying the competitive landscape—from Ventyx and NodThera to BioAge and Insilico Medicine—racing to capitalize on what is now one of pharma’s most strategically significant targets. The results are still early and this report is for information purposes only.
Motivation Behind this Post and Conflict of Interest: Potentially Best-in-Class CNS-penetrant NLRP3 Inhibitor Designed Using Generative AI, Multiparameter Optimization, and Massively-Parallel Experimentation
The main motivation for this post is that I am genuinely excited about this protein target. It sits on the intersection of multiple hallmarks of aging and is probably the most promising target for inflammaging. But I also have major conflict of interest here since Insilico is also developing the brain-penetrant NLRP3i that just completed IND-enabling studies. Our business model is to pick the most promising and impactful targets using AI, predominantly those that are implicated in aging and disease at the same time, take them to developmental candidate stage (DC) or even into the clinic and then license to the pharmaceutical companies. And while we like to focus on novel targets, often we wait until other companies and academics try the target in a variety of preclinical and clinical models and then devise a strategy to unlock the full potential of the target by designing the molecule with unique features that would make it as close to a perfect drug as possible. And since we have many years of experience using Chemistry42 generative AI platform, automation and established network of partner research labs, we can perform multiparameter optimization to achieve unique properties such as increased safety, activity, oral administration, selectivity, brain-penetration, gut-restriction, multi-targeting, and many other properties. In the NLRP3i project, our objective was to use the full potential of generative AI to design, multiparameter optimization, and parallel experimentation to design several series of molecules with the highest possible levels of safety, especially liver safety, which we think is most important in chronic diseases, and high level of brain penetration. Recent story of Danuglipron in the GLP-1 space, where Danuglipron failed in a Phase 3 study due to the liver toxicity signal while the competing Orfoglipron successfully completed Phase 3 serves as a great example for the need to prioritize safety over any other molecular property.
Originally, I was not planning to purpose NLRP3i for obesity as a primary indication, since we believe the target is more likely to succeed in Parkinson’s disease and since it has long patent life, it can later be repurposed for many other indications including Alzheimer’s and potentially into chronic conditions like obesity, muscle wasting, and even chronic pain. But clinical results in obesity increase the attention to this target manyfold since most of the pharmaceutical companies are now working on their cardiometabolic portfolios while some of the pharma companies with CNS franchises that would make perfect partners for this drug may be a bit slower when making decisions and execute on in-licensing. So increased levels of attention to NLRP3 in the obesity space definitely help me partner this potentially best-in-class CNS-penetrant molecule. In my opinion, this is one of the best molecules and targets for longevity and every big pharma serious about longevity (cardiometabolism, CNS, I&I and fibrosis) needs to have one.
Landmark Phase 2 Results: A New Pillar of Cardiometabolic Health
The clearest indication that NLRP3’s moment may have arrived comes from Ventyx’s Phase 2 trial of their oral NLRP3 inhibitor, VTX3232, in 175 patients with obesity and high cardiovascular risk. The topline results were striking: patients treated with VTX3232 experienced a nearly 78% reduction in high-sensitivity C-reactive protein (hsCRP) levels after 12 weeks. hsCRP is a key marker of systemic inflammation and an independent predictor of heart disease.
The results propelled Ventyx to new highs (>250% increase over 12 months) and resulted in significant increase in pharma interest in brain-penetrant NLRP3i in general. Just in case, I am not an investor in Ventyx and this post does not represent any form of investment advice.
We recently put a cool preprint on NLRP3i online without any serious promotion (as we plan to have it go through peer-review) and the number of downloads has increased considerably - pharma is definitely getting interested.
Ventyx’s results draw significant attention for many reasons. The drug also drove down interleukin-6 (IL-6)—another inflammatory cytokine closely tied to cardiovascular risk—to a median of 1.6 ng/L, below the 1.65 ng/L threshold associated with lower risk of cardiovascular events. Beyond these primary inflammatory markers, the trial documented statistically significant improvements in multiple cardiometabolic biomarkers. Levels of lipoprotein(a)—a notoriously hard-to-modify genetic risk factor for atherosclerosis—dropped meaningfully. So did fibrinogen and erythrocyte sedimentation rate (ESR), general indicators of inflammatory activity.
One outcome was conspicuously absent: weight loss. Ventyx’s NLRP3 inhibitor did not cause patients to lose weight on its own or in combination with the GLP-1 agonist semaglutide. While this might seem like a miss, it highlights a crucial scientific observation: NLRP3 inhibitors appear to target a different pathological axis (inflammation) than GLP-1 drugs (which act on appetite and insulin sensitivity). This finding helps clarify that the residual inflammatory risk that can persist even after weight loss is an independent and potentially druggable target.
Rather than competing with GLP-1s, these agents may be positioned to complement them. The Ventyx data showed that adding VTX3232 to semaglutide produced significant additional reductions in hsCRP, IL-6, and Lp(a) over semaglutide alone. This suggests a potential new paradigm where physicians might one day use a triad of therapies: one for lipids (statins), one for metabolism (GLP-1s), and one for inflammation (NLRP3 inhibitors).
The ‘Pipeline in a Product’: NLRP3’s Versatility Across Human Disease
The excitement isn’t limited to cardiometabolic health. The reason NLRP3 is considered one of the most important targets in pharma is its incredible versatility. It acts as a central sensor for a wide array of “danger signals” that are understood to drive pathology across multiple organ systems.
Neurodegenerative Diseases: In the brain, NLRP3 is activated in microglia by protein aggregates like amyloid-beta (in Alzheimer’s) and alpha-synuclelin (in Parkinson’s), which may unleash the chronic neuroinflammation that contributes to neuronal damage.
Atherosclerosis: In our arteries, NLRP3 is thought to be activated by cholesterol crystals, triggering the vascular wall inflammation that initiates and propagates atherosclerotic plaques.
NASH (Fatty Liver Disease): In the liver, NLRP3 activation is considered a key event that drives the progression from simple fatty liver to the more dangerous inflammatory state of non-alcoholic steatohepatitis (NASH) and fibrosis.
Gout: Gouty arthritis is a classic NLRP3-driven disease. The painful flares are caused by the activation of the inflammasome in response to monosodium urate crystals in the joints.
Autoimmune and Autoinflammatory Diseases: The very discovery of NLRP3 was rooted in rare genetic autoinflammatory syndromes. Its dysregulation is now implicated in a host of more common autoimmune conditions, including rheumatoid arthritis.
Kidney Disease: In the kidneys, NLRP3 activation may contribute to the inflammation and fibrosis that characterize chronic kidney disease.
This breadth is notable. A single, well-tolerated, and effective oral NLRP3 inhibitor could have applications spanning cardiology, neurology, rheumatology, hepatology, and nephrology, representing a significant opportunity.
Parkinson’s Disease: An Anchor Indication with Growing Momentum
If obesity and heart disease represent one major front for NLRP3 inhibitors, Parkinson’s disease is fast becoming another. The new strategy in neurodegeneration is to target neuroinflammation, where NLRP3 is believed to be a master controller.
Recent clinical evidence lends support to this approach. Earlier this year, NodThera announced results from a Phase 1b/2a trial of its brain-penetrant inhibitor, NT-0796, in Parkinson’s patients. Over just 28 days, key inflammatory biomarkers in patients’ cerebrospinal fluid (CSF)—including IL-1β and IL-6—were significantly reduced. Intriguingly, markers of neuron damage (NfL) and microglial activity (sTREM2) also declined. As Alan Watt, President and CSO of NodThera, noted, “These clinical results are striking, showing for the first time in Parkinson’s disease patients that we can effectively inhibit NLRP3 activity in the brain and reduce markers of neuroinflammation.”
This is not an isolated finding. Ventyx also tested its brain-penetrant VTX3232 in a Phase 2a study in early Parkinson’s patients. The results were also compelling, showing robust drug penetration into the CSF and reductions of inflammatory cytokines IL-1β and IL-18 by 14% to 52%. The trial also reported a statistically significant improvement in both motor and non-motor symptoms on the validated MDS-UPDRS scale. It is important to note that this was a small, open-label study, and these preliminary observations require confirmation in larger, placebo-controlled trials.
The industry is taking notice. Roche is advancing its own inhibitor, selnoflast, in Phase 1b trials for Parkinson’s. And AI-driven biotechs like Insilico Medicine have completed IND-enabling studies for their candidate, ISM8969, an AI-designed molecule optimized for brain penetration, which showed dose-dependent improvements in motor function in preclinical models.
A Hotbed of Innovation and Competition
The NLRP3 “arms race” is fully underway, with each player differentiating their strategy.
Ventyx Biosciences has established a clear path in cardiometabolic disease, focusing on reducing cardiovascular risk independent of weight loss.
NodThera is pursuing a dual-pronged approach with its brain-penetrant NT-0796, targeting both neuroinflammation in Parkinson’s and hypothalamic inflammation as a potential driver of obesity.
BioAge Labs, a longevity-focused company, is also betting on a brain-penetrant inhibitor, BGE-102, rooted in human longevity data that links lower NLRP3 activity to healthier aging.
Insilico Medicine is leveraging its generative AI platform to create what it hopes will be a best-in-class, brain-penetrant molecule, ISM8969, initially targeting Parkinson’s but with a broad indication strategy.
The common thread is that NLRP3 is viewed as a franchise-level target. The competition is fierce, which means robust investment, rapid data generation, and a push to solve challenges through innovative chemistry and technology.
A Target Poised to Transform Aging-Related Disease
From systemic inflammation in obese patients to neuroinflammation in Parkinson’s, NLRP3 is proving to be a linchpin in disease processes across the board. The recent clinical results are a watershed moment, demonstrating in patients what scientists have hypothesized for years: tamping down the NLRP3 inflammasome can dramatically improve markers of health and may have the potential to alter disease trajectories.
In strategic terms, NLRP3 has become one of the most prized targets in pharma. Any company with aspirations in cardiometabolic disease, neurodegeneration, or longevity medicine will likely need a position on NLRP3. The reason is simple: NLRP3 isn’t tied to just one disease; it appears to be a fundamental node in the biology of aging and chronic illness.
We are witnessing the dawn of the anti-inflammaging era. The concept of treating aging-related chronic diseases at their inflammatory root is transitioning from aspirational theory to clinical reality. If NLRP3 is indeed a master switch of inflammaging, then modulating it could usher in a new era of preventive medicine—one in which we not only add years to life, but life to years, by keeping the destructive flames of chronic inflammation at bay.


“Anti-inflammaging era” - love that phrase. If NLRP3 really is the master switch, we might be witnessing the most promising scalable attempt to tackle aging at its inflammatory root
Thanks for the excellent info, Alex. In the meantime, natural anti-inflammatories such as curcumin, gingerol, capsaicin, and allicin among others, seem to be safe and effective alternatives.