Beyond GLP-1: Is NLRP3 the Next Trillion Dollar Target?
This 24-year-old target demonstrates promise across multiple chronic diseases and has been prioritized by two AI-driven longevity companies. The quality of the chemistry will decide the winner.
With over 15 years in target discovery and several dozen research papers under my belt—most of them centered on novel targets implicated in both aging and disease—I've long been a champion of target novelty above all else. In my earlier days, I was adamant that novelty was king: we should pour our efforts into uncharted territories and steer clear of anything familiar. This conviction was further supported by the questions from target discovery specialists who often chose our platforms that could pick absolutely novel targets. But experience has a way of reshaping perspectives. I've come to see that even well-established targets can unlock extraordinary potential when paired with the right chemistry. Drug discovery is a marathon, not a race, and being the first is often not the winning strategy. Take GLP-1, arguably the most profitable and transformative target in medical history—it was discovered more than 40 years ago, yet it's only now hitting its stride in revolutionizing treatments for obesity and beyond. Or consider TYK2, an "ancient" target that's suddenly buzzing with promise, powering over a dozen clinical trials at Takeda alone. This begs the question: How do we uncover the next GLP-1 or TYK2—targets that could prove even more pivotal in the quest for longevity?
The Master Switch Every Pipeline Needs
In the landscape of modern drug development, few targets offer the strategic value of a true "master switch"—a central node in human pathology so fundamental that controlling it promises not just a single product, but a generational franchise. For decades, the industry has chased the downstream consequences of chronic disease. We have lowered cholesterol, managed blood sugar, and cleared protein plaques, all while a common fire has smoldered beneath: a low-grade, persistent, sterile inflammation now known as "inflammaging". This process is the acknowledged driver of the majority of chronic diseases, from atherosclerosis and Alzheimer's to type 2 diabetes and cancer.
The strategic imperative for any forward-looking R&D organization is clear: find and drug the source of the fire. The leading candidate for this master switch—and arguably the most valuable target of the next decade—is the NLRP3 inflammasome. NLRP3 is not merely another inflammatory mediator; it is a primary sensor of the very cellular debris, metabolic stress, and sterile danger signals that define aging and chronic disease. Its dysregulation is implicated in a breathtakingly broad array of human pathologies, making it one of the most intensely studied targets today.
This is not a speculative bet. The target is validated by human genetics, de-risked by successful downstream drugs, and backed by an explosion in public research funding. The race to develop NLRP3 inhibitors is on, with big pharma placing billion-dollar bets through acquisitions and next-generation biotechs deploying advanced platforms to create best-in-class assets.
This report will make the case that for any major pharmaceutical company, a competitive NLRP3 program is no longer optional—it is a strategic necessity. We will demonstrate that NLRP3 inhibitors represent a foundational, franchise-level opportunity with the potential to become the next GLP-1 class, and that companies leveraging cutting-edge technology to design superior molecules, such as Insilico Medicine's AI-optimized inhibitor, are positioned to dominate this transformative field.
History of NLRP3: From Rare Disease to a Unifying Theory of Inflammation
The story of NLRP3 began, as many great drug discovery stories do, with rare diseases. In 2001, Dr. Hal Hoffman and his colleagues identified mutations in a gene they named CIAS1 (now NLRP3) as the cause of two rare genetic fever syndromes: familial cold autoinflammatory syndrome and Muckle-Wells syndrome. This discovery of the protein, dubbed "cryopyrin," was the first definitive proof that a single protein could act as a powerful, autonomous trigger for systemic inflammation.
The conceptual framework arrived shortly after. In 2002, the laboratory of the late Jürg Tschopp introduced the term "inflammasome"—a large, intracellular protein complex that activates inflammatory cytokines—and by 2004, had identified NLRP3 as a central sensor in this complex. These pioneering efforts provided two critical pillars for drug development:
Irrefutable Human Genetic Validation: The direct, causal link between gain-of-function mutations in the NLRP3 gene and the severe inflammatory diseases now known as Cryopyrin-Associated Periodic Syndromes (CAPS) provided unambiguous validation of the target.
Immediate Pharmacological Proof-of-Concept: Drugs that block IL-1β, the primary cytokine product of NLRP3 activation, proved to be transformative therapies for CAPS patients, confirming the pathway was druggable.
The paradigm shifted when researchers, including Tschopp and Eicke Latz, realized NLRP3 was not just for rare diseases. They discovered it could be activated by a vast array of common, sterile danger signals: the monosodium urate crystals that cause gout, cholesterol crystals in atherosclerotic plaques, and amyloid-β aggregates in Alzheimer's disease. NLRP3 was no longer just the cause of rare inflammasomopathies; it was the central alarm for a vast plethora of diseases.
In 2015, a final piece of the puzzle fell into place when Dr. Luke O'Neill and colleagues identified MCC950, the first potent, small-molecule inhibitor of NLRP3. This tool compound ignited a boom in drug development, allowing researchers to probe the pathway's function with precision and inspiring the race to create clinical-grade inhibitors that is now in full force.
A Decade of Explosive Research Activity
The commercial rationale for pursuing NLRP3 is built on a foundation of exponentially growing scientific validation. A review of publication and grant funding data reveals a target that has moved from a niche interest to a global research priority, de-risking the field for private investment.
Publication Surge: In the early 2010s, papers on the NLRP3 inflammasome were a trickle. Today, they are a flood. Across all fields, publications have grown exponentially since 2013. In neurology alone, annual publications grew from single digits before 2013 to nearly 300 in 2021. In cardiovascular disease, publications peaked at 94 in 2022, with a total of 516 articles between 2012 and 2023. In myocardial infarction research, publications grew from just two in 2013 to 61 in 2023. This torrent of research, dominated by institutions in China and the United States, has firmly established NLRP3 as a centerpiece of modern inflammation biology.
Massive NIH Investment: The US National Institutes of Health (NIH) has poured resources into NLRP3 research, signaling a major strategic priority. A query of the NIH RePORTER database shows that the number of active, funded projects containing the term "NLRP3" has more than tripled in just five years, from 392 in fiscal year 2020 to 1,326 in 2024. This funding is not siloed; it spans a wide range of institutes, including the National Institute on Aging (NIA), the National Institute of Neurological Disorders and Stroke (NINDS), and the National Heart, Lung, and Blood Institute (NHLBI), reflecting the target's vast therapeutic potential. Philanthropic organizations like the Michael J. Fox Foundation have also awarded grants to test NLRP3 inhibitors in Parkinson's models. This publicly funded research provides an invaluable foundation, dramatically lowering the risk for pharmaceutical development.
The number of reviews implicating NLRP3 in every age-related, inflammation-related and immune-related disease is huge. Just search “The role of NLRP3 in diseases” will give you several dozen beautiful images and even AI-generation performed above, while not perfect, shows the broad therapeutic potential of this target.
NLRP3 Inhibitors: The Players and the Clinical Battleground
The scientific gold rush has triggered a parallel race in the biopharma industry. Today, at least 20 companies are advancing over 25 distinct NLRP3 inhibitors, with programs spanning from preclinical discovery to Phase 3 trials. The strategic landscape is defined by high-value acquisitions and a clear bifurcation between peripherally-restricted and brain-penetrant assets.
Big Pharma's Billion-Dollar Votes of Confidence:
The surest sign of a target's perceived value is when large pharma companies enter via acquisition, placing billion-dollar bets on the future of the class.
Roche: In 2020, Roche paid €380 million ($451 million) upfront to acquire Inflazome and its clinical-stage NLRP3 inhibitors, including the brain-penetrant inzomelid and peripherally-restricted somalix (now selnoflast). This followed their 2018 acquisition of Jure Therapeutics for its NLRP3 assets.
Novartis: In 2019, Novartis acquired IFM Tre for $310 million upfront and up to $1.265 billion in milestones, gaining a portfolio that included the clinical-stage systemic antagonist DFV890.
Novo Nordisk: In a strategically critical move, the leader in GLP-1s licensed Ventus Therapeutics' peripherally-restricted NLRP3 inhibitor (now NNC6022-0001) in a 2022 deal worth up to $700 million, signaling their belief that NLRP3 inhibition is a key complementary mechanism for cardiometabolic diseases.
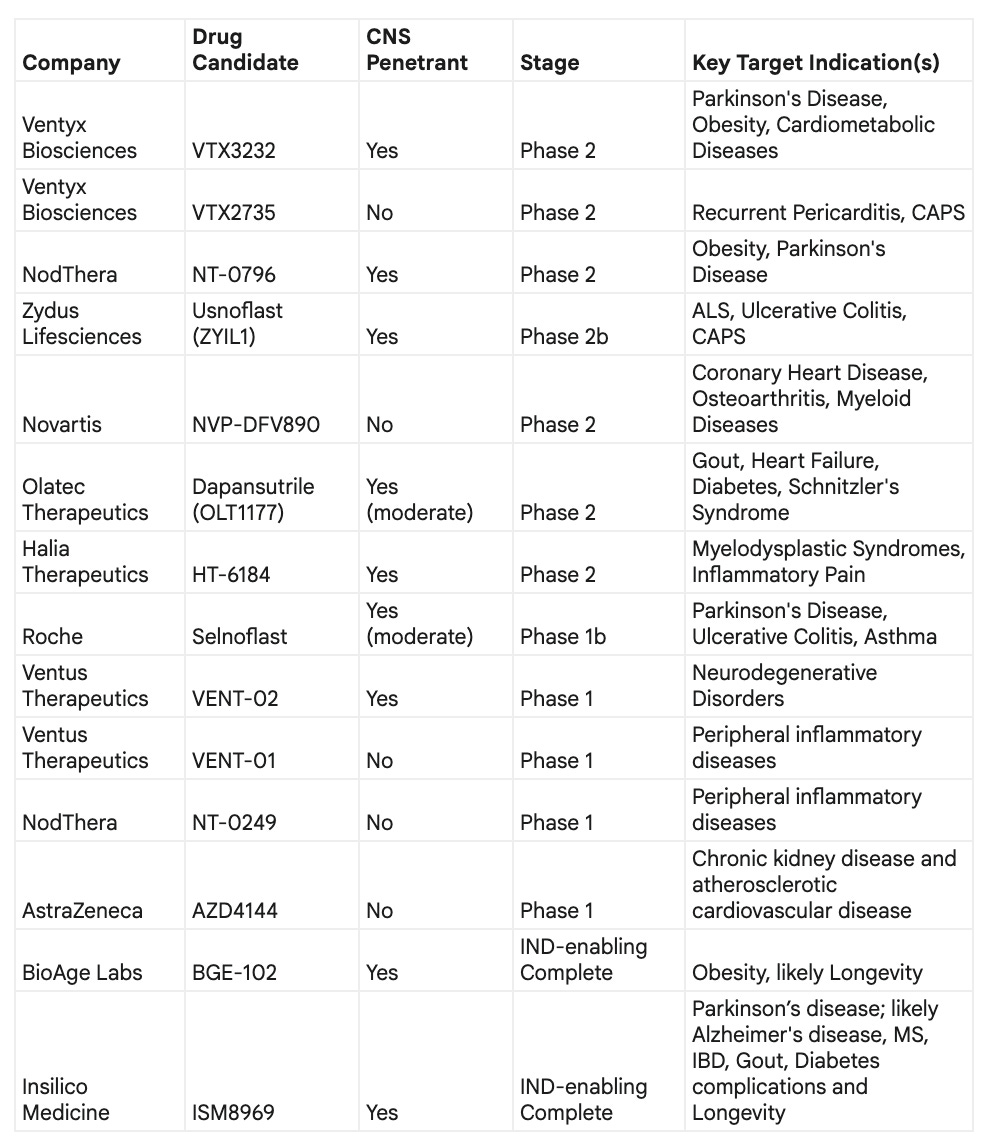
The following table provides a snapshot of the competitive clinical pipeline:
* the table was compiled using the LLM and may contain inaccuracies; perform your own research
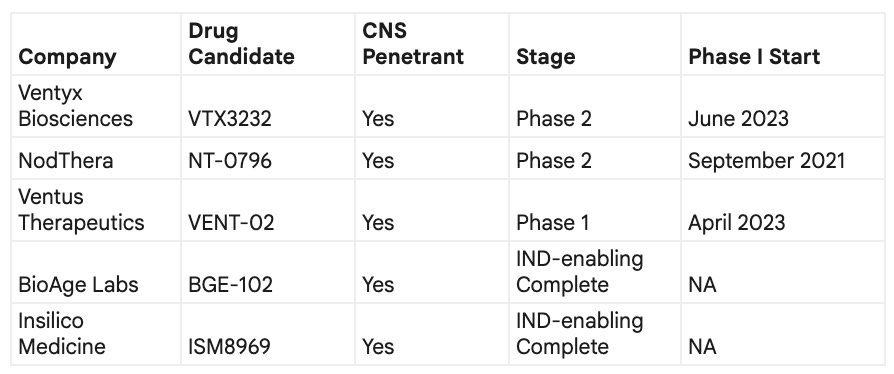
While it may seem to be a very crowded space, first entrants did not optimize their chemistry to unlock the maximum potential of this magical target. The main competitors in this race as far as I can see are the ones that optimized for higher level of safety and brain penetration. If we look at the Phase I start dates, there is a good chance for best-in-class molecular structures with highly differentiated properties to catch up and take advantage of this unique market opportunity with GLP-1-like potential.
* the table was compiled using the LLM and may contain inaccuracies; perform your own research
Unsurprisingly, even if I were not deeply conflicted here, my bet would be on Insilico’s compound. Insilico’s experienced molecular designers with massively-parallel laboratory and animal validation developed multiple praised assets. The USP1 inhibitor which had other frontrunners in the past and was out-licensed in early stages with $80 million cash upfront, is likely not only best-in-class but may also be first-in-class since other inhibitors did not do so well. The NLRP3 inhibitor was designed more recently using even more sophisticated next-generation tools and validation workflows and has every chance of being the best.
The Patent Landscape: Like with GLP-1s, Being the Best in Many Big Indications is More Important Than Being the First in Small Indications
For any executive evaluating the NLRP3 space, the intellectual property landscape is as critical as the clinical data. The field shows a clear evolution, with foundational patents for some of the more advanced clinical candidates in Phase II dating back as far as 2007. In stark contrast, a new wave of highly differentiated and improved molecules has emerged, protected by fresh composition of matter patents with priority dates in 2023 and 2024. This creates a remarkable 16- to 17-year advantage in patent life for these next-generation assets. From a strategic standpoint, this is critical. As the first-generation molecules continue to de-risk the target by demonstrating efficacy across a range of indications, the newer, substantially improved molecules will be positioned to enter the market and cover a massive number of indications with a much longer and more profitable period of market exclusivity. Some of the new molecules were optimized with the knowledge of the liabilities of the earlier molecules, and this is where, IMHO, Insilico’s ISM8969 NLRP3 inhibitor stands out to facilitate for massive indication expansion into broad indications.
A Safer Way to Tame Inflammation
The ultimate value of NLRP3 inhibitors lies in their potential for a superior safety profile. For decades, anti-inflammatory therapies have been sledgehammers—corticosteroids, TNF inhibitors, and JAK inhibitors—that broadly suppress the immune system. While effective, this approach carries a heavy burden of infection risk, confining their use to severe diseases and making them unsuitable for chronic, preventative care.
NLRP3 inhibitors offer a paradigm of precision. By selectively blocking only the NLRP3 inflammasome, they are designed to quell the pathological sterile inflammation that drives chronic disease while leaving other crucial arms of the immune system—like the NLRP1, NLRC4, and AIM2 inflammasomes needed to fight infection—fully intact. This targeted approach promises to uncouple efficacy from the risk of broad immunosuppression.
Early development was not without hurdles. The first-generation inhibitor, MCC950, was halted due to liver toxicity, as was another early candidate, GDC-2394. However, this was determined to be an off-target effect of a specific chemical scaffold, not an on-target liability. The new generation of structurally diverse inhibitors has shown an encouraging safety profile in clinical trials, with no major issues reported to date for candidates from Ventyx, NodThera, and Olatec even though it is still early days. If this safety profile holds, it will unlock the use of these drugs for the largest chronic disease markets.
NLRP3 and the Biology of Aging: The Longevity Prize
For the most forward-thinking biotechs, NLRP3 is more than a target for specific diseases; it is a target for aging itself. The chronic, low-grade "inflammaging" that accompanies aging is a key driver of nearly all age-related decline, and NLRP3 is its central engine. It senses the accumulated damage of aging—misfolded proteins, mitochondrial dysfunction, metabolic byproducts—and translates it into persistent, tissue-damaging inflammation.
The evidence is stunning: genetically deleting the Nlrp3 gene in mice extends their mean healthspan and lifespan by up to 34% and maximum lifespan of 29%. This makes NLRP3 one of the most potent longevity targets ever identified. It is no surprise, then, that some of the world's most advanced longevity biotechnology companies have independently made NLRP3 a top priority. BioAge, one of the pioneers in longevity biotechnology and a long-term supporter of the Aging Research and Drug Discovery meeting, prioritized NLRP3 as the lead program in their longevity-centric pipeline.
The Two Biotechs Betting Big on NLRP3: BioAge and Insilico
The convergence of two philosophically distinct, next-generation biotechs on NLRP3 provides the strong signal of the target's fundamental importance in longevity.
BioAge Labs: Human Data First
BioAge's platform is grounded in the deep analysis of longitudinal human aging data to find pathways correlated with healthy longevity. Their analysis identified lower NLRP3 activity as a key factor in obesity directly prompting it as their lead program.
The Drug: BGE-102, a potent, novel, brain-penetrant NLRP3 inhibitor.
The Indication: Obesity, targeting the hypothalamic inflammation that drives appetite dysregulation. Preclinical data shows BGE-102 monotherapy produces weight loss comparable to semaglutide, with additive effects in combination.
Status: IND submission is planned for mid-2025, with initial Phase 1 SAD data expected by year-end.
Insilico Medicine: Generative AI and the 'Faberge Egg' Philosophy
Insilico Medicine employs Pharma.AI, a sophisticated end-to-end artificial intelligence platform, to identify novel targets and design novel molecules with optimized properties from scratch. Their business model requires that every program is designed for partnership and must be a "Faberge egg"—an asset engineered to be as close to perfect as possible. For a potential pharmaceutical partner, any flaw in potency, selectivity, or safety could jeopardize a deal. This philosophy is embodied in their NLRP3 program, which they consider a crown jewel of their pipeline.
The Drug: ISM8969, a novel, AI-designed, brain-penetrant preclinical candidate.
The Indication Strategy: A broad-spectrum approach targeting a range of inflammation-related conditions where ISM8969's properties give it a competitive edge, including Parkinson disease, Alzheimer's disease, gout flare, IBD, and diabetes complications. Of course, it is also developed as a longevity therapeutic.
The Strategic Advantage: While the primary design objective for ISM8969 was an unparalleled long-term safety profile, Insilico's AI platform managed to achieve much more. It simultaneously optimized for high potency, exquisite selectivity against other inflammasomes, and ideal brain-penetrant properties, creating a molecule designed from the ground up to be a best-in-class candidate. This ability to engineer superior, multi-parameter assets combined with the autonomous validation lab, established laboratory infrastructure and experience delivering 22 developmental candidates with 10 reaching clinical stage and 4 partnered assets in clinical trials is a strong signal of ISM8969 superior properties.
Could NLRP3 Inhibitors Be the Next GLP-1?
The commercial potential of the NLRP3 inhibitor class is staggering, drawing direct comparisons to the GLP-1 agonists, which are on track to become the best-selling drug class in history. The investment case rests on three pillars:
The "Pipeline in a Product": NLRP3 inhibitors are the ultimate "pipeline in a product". Because aberrant NLRP3 activation drives so many conditions, a single successful drug could secure approvals in numerous multi-billion dollar markets, including neurodegeneration (Alzheimer's, Parkinson's), cardiometabolic diseases (atherosclerosis, NASH, obesity, kidney disease), and autoimmune disorders (gout, IBD). The total addressable market is estimated to be well over $100 billion.
Synergy with GLP-1s: The most compelling near-term opportunity is obesity. CNS-penetrant NLRP3 inhibitors can address hypothalamic inflammation, a root cause of metabolic dysregulation. Crucially, they are not just competitors to GLP-1s but essential partners. Preclinical data from both NodThera and BioAge show that combining an NLRP3 inhibitor with a GLP-1 agonist leads to greater weight loss, better body composition (preserving muscle), and prevention of weight regain after stopping the GLP-1. This positions NLRP3 inhibitors as a "must-have" add-on to enhance and sustain the effects of the current $100B+ obesity market leaders.
The Statin Analogy—A Future Preventive Medicine: The ultimate potential is preventative. Just as statins treat the risk factor of high cholesterol, NLRP3 inhibitors could treat the foundational risk factor of chronic inflammation. With a strong long-term safety profile, a daily oral NLRP3 inhibitor could become the "new statin," taken by millions to prevent or delay the entire spectrum of age-related diseases.
The Anti-Inflammaging Era is Here
It is rare to find a target that sits at the nexus of validated human genetics, massive unmet medical need, and the fundamental biology of aging. NLRP3 is that target. The scientific consensus is clear, the commercial momentum is undeniable, and the therapeutic potential is transformative.
The development of NLRP3 inhibitors marks a paradigm shift from reactive, single-disease treatment to a proactive strategy that targets the root cause of why we get sick as we age. For pharmaceutical leadership, the message is unequivocal: an NLRP3 program is not a speculative venture; it is a foundational pillar for future growth. Inaction is not a neutral stance; it is a strategic liability. The companies that will win are those with differentiated, best-in-class assets. In this crowded field, the winning asset will not be just another "me-too" inhibitor; it will be a molecule engineered with flawless precision—a true "Faberge egg" designed for maximum efficacy and uncompromising safety. This is where technology becomes the ultimate arbiter of success. Platforms like Insilico Medicine's generative AI, which can deliver these highly optimized assets like their crown jewel NLRP3 inhibitor, ISM8969, offer the most direct path to market leadership, secured by one of the most durable patent estates in the entire field.
Based on the overwhelming evidence, the conclusion is simple. If a safe and effective NLRP3 inhibitor becomes available, it would be a cornerstone of modern medicine. For anyone serious about extending not just lifespan but healthspan, it is a drug worth taking. The magic of NLRP3 is that it is poised to make the dream of anti-aging medicine a clinical reality.
Note that this article was prepared using generative AI and may contain hallucinations, inaccuracies, and errors. Please do your own research. While the author is the CEO of Insilico Medicine, the statements and view presented in Forver.ai do not represent the views and opinions of Insilico Medicine.
Further Reading
The NLRP3 Inflammasome as a Critical Actor in the Inflammaging Process https://pmc.ncbi.nlm.nih.gov/articles/PMC7348816/
Aging-Associated TNF Production Primes Inflammasome Activation and NLRP3-Related Metabolic Disturbances https://journals.aai.org/jimmunol/article/197/7/2900/109369/Aging-Associated-TNF-Production-Primes
NLRP3 inflammasome plays a vital role in the pathogenesis of age-related diseases in the eye and brain https://www.researchgate.net/publication/375778874_NLRP3_inflammasome_plays_a_vital_role_in_the_pathogenesis_of_age-related_diseases_in_the_eye_and_brain
The role of NLRP3 inflammasome in aging and age-related diseases https://www.researchgate.net/publication/377980181_The_role_of_NLRP3_inflammasome_in_aging_and_age-related_diseases
Targeting the NLRP3 inflammasome in chronic inflammatory diseases: current perspectives https://www.dovepress.com/targeting-the-nlrp3-inflammasome-in-chronic-inflammatory-diseases-curr-peer-reviewed-fulltext-article-JIR
Biological and therapeutic significance of targeting NLRP3 inflammasome in the brain and the current efforts to develop brain-penetrant inhibitors https://pmc.ncbi.nlm.nih.gov/articles/PMC11955958/
Inhibitors of the NLRP3 inflammasome pathway as promising therapeutic candidates for inflammatory diseases (Review) https://pmc.ncbi.nlm.nih.gov/articles/PMC10049046/
Could an NLRP3 inhibitor be the one drug to conquer common diseases? https://cen.acs.org/pharmaceuticals/drug-discovery/Could-an-NLRP3-inhibitor-be-the-one-drug-to-conquer-common-diseases/98/i7
Deciphering NLRP3 Inhibitors and Keeping Up with Their Recent Developments https://synapse.patsnap.com/blog/deciphering-nlrp3-inhibitors-and-keeping-up-with-their-recent-developments
The discovery of NLRP3 and its function in cryopyrin-associated periodic syndromes and innate immunity https://pmc.ncbi.nlm.nih.gov/articles/PMC10950545/
The inflammasome: in memory of Dr. Jurg Tschopp https://pmc.ncbi.nlm.nih.gov/articles/PMC3252823/
Screening NLRP3 drug candidates in clinical development: lessons from existing and emerging technologies https://pmc.ncbi.nlm.nih.gov/articles/PMC11345644/
The NLRP3 inflammasome in health and disease: the good, the bad and the ugly https://pmc.ncbi.nlm.nih.gov/articles/PMC3193914/
First‐in‐human phase 1 trial evaluating safety, pharmacokinetics, and pharmacodynamics of NLRP3 inflammasome inhibitor, GDC‐2394, in healthy volunteers https://pmc.ncbi.nlm.nih.gov/articles/PMC10499406/
Advances in inflammasome research: Recent breakthroughs and future hurdles https://pmc.ncbi.nlm.nih.gov/articles/PMC7678016/
Emerging trends and hot spots of NLRP3 inflammasome in neurological diseases: A bibliometric analysis https://pmc.ncbi.nlm.nih.gov/articles/PMC9490172/
Frontiers and Hotspot Evolution of NLRP3 Inflammasome in Myocardial Infarction Research: A Bibliometric Analysis From 2013 to 2024 https://pmc.ncbi.nlm.nih.gov/articles/PMC11865457/
The research trends and hotspots of NLRP3 inflammasome in Alzheimer's disease: a bibliometric and visualization analysis https://www.medrxiv.org/content/10.1101/2025.01.08.25320239v1.full.pdf
Inhibition of the NLRP3 inflammasome improves lifespan in animal murine model of Hutchinson–Gilford Progeria https://www.embopress.org/doi/10.15252/emmm.202114012
Pharmacological Inhibitors of the NLRP3 Inflammasome https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2019.02538/full
Roche pays €380M for NLRP3 biotech Inflazome, claiming a leading position in hot field https://www.fiercebiotech.com/biotech/roche-pays-eu380m-for-nlrp3-biotech-inflazome-claiming-a-leading-position-hot-field
A hefty price tag for NLRP3 https://www.drugdiscoverynews.com/a-hefty-price-tag-for-nlrp3-13292
21st Century Miracle Drugs: Spotlight on Clinical NLRP3 Inflammasome Inhibitors https://drug-dev.com/inglammasome-inhibitors-21st-century-miracle-drugs-spotlight-on-clinical-nlrp3-inflammasome-inhibitors/
The NLRP3 Inflammasome as a Pathogenic Player Showing Therapeutic Potential in Rheumatoid Arthritis and Its Comorbidities: A Narrative Review https://www.mdpi.com/1422-0067/25/1/626
NodThera NLRP3 inhibitor nearly matches Wegovy for weight loss https://www.fiercebiotech.com/research/nodtheras-clinical-nlrp3-inhibitor-almost-matches-wegovy-weight-loss-mice-bonus-heart
Ventus Therapeutics Enters Exclusive Development and License Agreement with Novo Nordisk for NLRP3 Inhibitor Program https://www.ventustx.com/ventus-therapeutics-enters-exclusive-development-and-license-agreement-with-novo-nordisk-for-nlrp3-inhibitor-program/


Thanks Alex for this insightful analysis of the Inflammasome and its relevance to human biology - especially now the potential of NLRP3 ... I remember highlighting this many years ago when I was part of Pharma - that was not taken seriously. Glad that it is now getting its day in the sun! Best Wishes to you and InSilico ... Ravi Kiron
Once again, a cheap and effective solution already exists:
Vitamin D acts as a crucial negative regulator of the pro-inflammatory NLRP3 inflammasome, primarily through its receptor (VDR) binding directly to NLRP3, blocking its activation, and inhibiting the release of inflammatory cytokines like IL-1β and IL-18. This interaction reduces oxidative stress and dampens excessive inflammation seen in conditions like asthma, diabetes, and COVID-19, highlighting vitamin D as a potential therapeutic target against NLRP3-driven diseases.