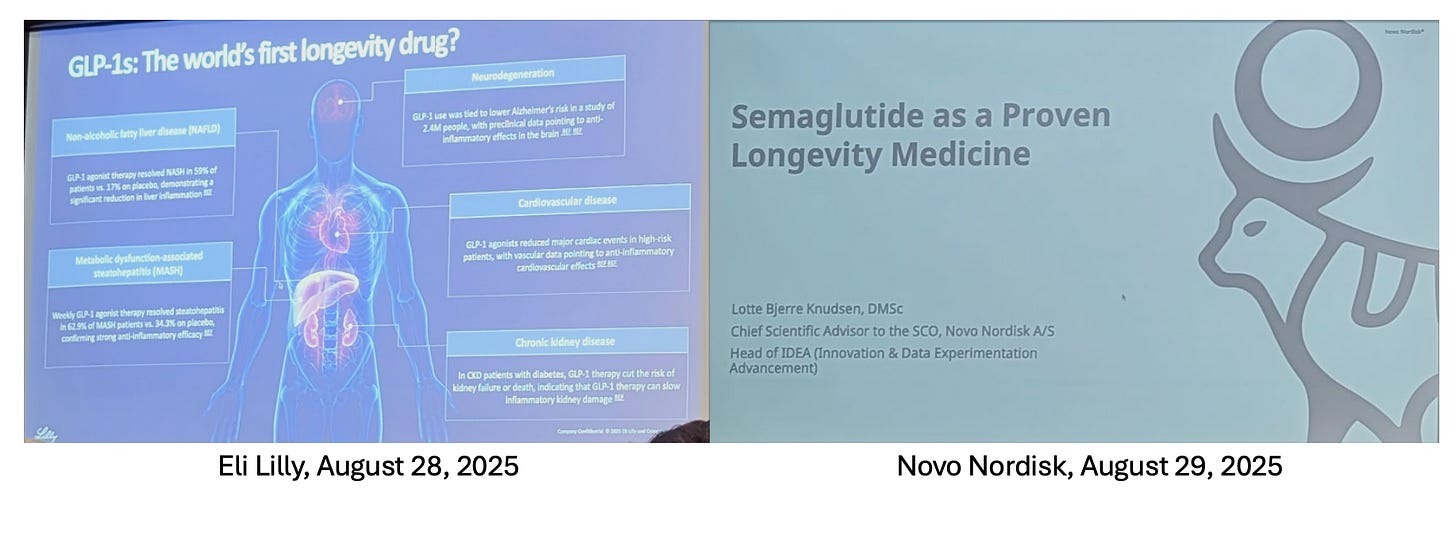
GLP-1s: The world's first longevity drug?
The "DeepSeek Moment" in the longevity biotechnology we've all been waiting for transpired on August 29, 2025 at the 12th ARDD meeting in Copenhagen
We are living through a pivotal moment in the history of the modern pharmaceutical industry. At the 12th Aging Research and Drug Discovery meeting (ARDD2025) in Copenhagen, the narrative surrounding GLP-1 receptor agonists shifted profoundly. In less than 24 hours, the two largest biopharmaceutical companies presented these blockbuster diabetes and obesity treatments as the world’s first recognized longevity therapeutics.
Dr. Andrew Adams of Eli Lilly giving a keynote talk at the 12th ARDD in Copenhagen
The atmosphere in the Grand Hall was electric. On Thursday, August 28th, Dr. Andrew Adams, Group Vice President of Molecule Discovery and Director of the Lilly Institutes of Genetic Medicine at Eli Lilly, concluded his presentation on the GLP-1 story with a seismic question: "Are GLP-1s the world's first longevity drug?"
It was a watershed moment. In an audience packed with senior R&D leaders—including over 10 from Eli Lilly, alongside key figures from Novo Nordisk, AstraZeneca, Roche, Boehringer Ingelheim, and others —the realization dawned that a traditional pharma giant had officially evolved into the first longevity pharma company.
The following day, the narrative was cemented. Dr. Lotte Bjerre Knudsen, the legendary Chief Scientific Advisor at Novo Nordisk and the driving force behind the development of semaglutide (recently recognized with the 2025 Breakthrough Prize), took the stage. Acknowledging the compelling evidence presented during the conference, she updated her title slide to an unequivocal declaration: "Semaglutide as a Proven Longevity Medicine."
Dr. Lotte Bjerre Knudsen giving a keynote talk at the 12th ARDD
This historic pivot signals that the longevity field has arrived in the mainstream.
The New Pharmaceutical Imperative
With strong evidence suggesting systemic benefits far beyond glycemic control and weight management—including cardiovascular protection and effects on inflammation—GLP-1s are now viewed as foundational longevity therapeutics.
This recognition changes the strategic landscape. It is now clear that every company serious about the future of medicine must have a versatile GLP-1 molecule in their portfolio.
The events at ARDD2025 have sparked urgent discussions among R&D leaders, all grappling with the same fundamental questions: What is the best way to discover and develop longevity therapeutics that will follow the GLP-1 story? What target should we choose next, what modality, which indication, and crucially, how do we measure the effect on aging using reliable aging clocks?
These are the central questions defining pharma strategy today. To address them, the field is rapidly developing new frameworks, as seen in recent perspective papers we published, discussing "clock-to-clock" analysis and using diseases as a testbed for longevity interventions.
The Next Frontier: The Oral Small Molecule GLP-1s
While injectable peptides like semaglutide and tirzepatide have revolutionized treatment, they are not the endgame. The future belongs to oral small molecules that are cheaper, easy to store and ship, combine, produce and administer.
However, developing an effective, safe, and tolerable oral small-molecule GLP-1 has proven incredibly challenging.
The journey toward an optimal oral GLP-1 is exemplified by the contrasting fates of Pfizer’s danuglipron and Eli Lilly’s orfoglipron.
Danuglipron demonstrated significant efficacy in weight reduction. However, its development faced major setbacks. The initial twice-daily formulation was discontinued due to exceptionally high rates of gastrointestinal adverse events (nausea and vomiting), leading to discontinuation rates exceeding 50% in trials. Ultimately, Pfizer discontinued the entire program in April 2025, citing that the risk-benefit profile, including a potential case of drug-induced liver injury (DILI) in a recent study, did not support further development.
Orfoglipron, a once-daily oral molecule, remains in successful late-stage development. While it also presents gastrointestinal side effects, its profile appears significantly more manageable and cleaner regarding liver safety signals, demonstrating a better balance of efficacy and tolerability.
These cases highlight the central challenge: ensuring exceptional safety and tolerability for long-term use.
Insilico’s "Mission Impossible": Designing the Ideal GLP-1 with AI For Once-a-Week Dosing
The ideal GLP-1 drug must achieve a delicate balance: an oral small molecule that is highly efficacious, possesses a superior safety and tolerability profile, and offers maximum convenience.
At Insilico Medicine, our focus has traditionally been on pursuing high-novelty targets, avoiding older, more established areas. However, the undeniable potential of GLP-1 as a longevity therapeutic, combined with the massive need for a better molecule, presented a unique challenge. We believed that with our generative chemistry capabilities and rapid experimentation, we could achieve the incredibly challenging chemical feat.
We decided to pursue "Mission Impossible": the most sophisticated, longevity-friendly GLP-1 design imaginable.
Our objective was precise and ambitious: an oral small molecule with a safety profile targeting even higher standards than orfoglipron, but critically, designed for once-a-week (QW) dosing. Achieving QW dosing in a small molecule requires extensive multi-parameter optimization to ensure sustained exposure and efficacy while minimizing side effects—a challenge perfectly suited for the battle tested generative AI. In this pursuit, the synergy between advanced generative AI, some of the world's best medicinal chemists, and rapid testing for safety and other properties is essential to getting to what we hope will be the world's best molecule.
At this stage we believe we achieved these unique properties with two amazing molecules one with the QW (once-a-week) dosing and another with QD (once-a-day) dosing and exceptional safety profile based on the currently available data. The initial evaluation of these molecules by the big pharma companies impressed some of the key GLP-1 veterans. We are now actively partnering our GLP-1 asset with QW properties, and the interest from big pharma has been overwhelming. The industry is actively seeking differentiated small molecule oral drugs that can overcome the limitations of current options.
This industry milestone is catalyzing a new era. We anticipate that almost every big pharma company will utilize GLP-1 alternatives as a base therapy, upon which other longevity therapeutics will be stacked. But our journey toward building the portfolio of safe small molecule oral longevity therapeutics is just beginning. Other novel and very well known protein and pathways are emerging as promising longevity targets. I believe that NLRP3, NR3C1, Amylin, Apelin, Activin and many other targets we described in our prior publications are poised to complement the effects of GLP-1, creating comprehensive anti-aging regimens.
We are witnessing a transformative moment in medicine. The age of longevity therapeutics has officially begun, and we are very happy to be in this field. It is, indeed, the best time to be alive.
Missed ARDD?
In case you missed the 12th ARDD but you would like to see the photos from this epic event, some of the Official Conference Photos are now online and here is my Personal Album from the conference (let me know if any of the photos should be removed). The conference was sold out a month in advance and we could not facilitate for more people due to capacity limits. Please make sure you register early for the 13th ARDD and put the last week of August into your calendars.
You will see the fun pictures from the bar (we served only light alcohol drinks). Did you know that if you sponsor the conference, you can design and name your own drink? I was very honest with mine - Zhavoronkov Aging Accelerator. Even this amount of alcohol is likely bad for you but let’s not waste time on this - it is a personal choice. But it seems like GLP-1s can help with that too according to clinical trials 😊




The whole metabolic disease epidemic in the United States occurred when our food supply changed rapidly, and we became sedentary.