Pharma, Biotechnology, and Longevity: 2025 Year in Review
Scientific Progress, Clinical Outcomes, and Regulatory Approvals
Precision Interventions & Small Molecule Renaissance
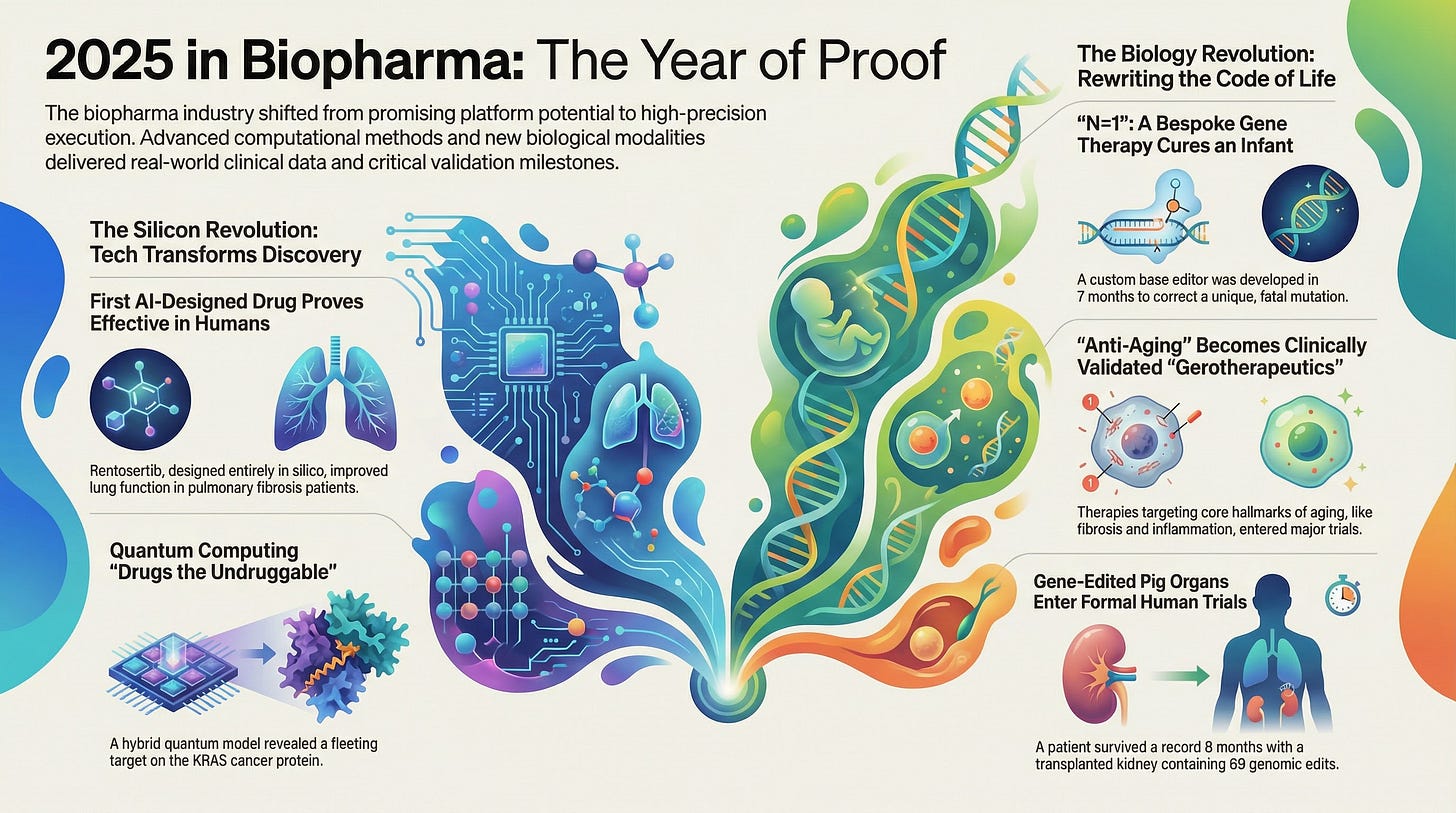
The year 2025 in biopharma was marked by precision breakthroughs across pharmaceuticals, biotechnology, and longevity research. The FDA approved 55 new drugs (46 novel drug entities), maintaining the high productivity of recent years. Notably, small-molecule medicines made a resurgence—comprising ~67% of approvals—after a period dominated by biologics. By contrast, cell and gene therapy approvals slowed (just 4 approvals, down from 7 in 2024), reflecting a consolidation phase in that field even as scientific progress accelerated. Overall, 32 orphan drugs and 31 first-in-class therapies gained approval, underscoring deep innovation. By the way, when I say 31 first-in-class therapies, I do not mean “absolutely novel”. Absolutely novel, we are talking about 5-7, depending on how you assess novelty. But even if we were talking 31 novel - this is still and incredibly low number. Just think about it - over $300 billion/year spent and only 31 novel drugs - we need to do better than that! On the positive side, China’s biotechnology industry has now caught up with the US and started investing in high-risk high-reward novel therapies. We need more countries to start investing in biotech and take risks for our benefit - at the end of the day, we are all patients.
2025 also saw multiple “firsts” translating cutting-edge biology into clinical reality. A personalized CRISPR gene edit saved a child’s life (”n=1” medicine in action). A T-cell–boosting immunotherapy validated Nobel Prize-winning regulatory T cell biology in humans. The first bispecific antibody showed unprecedented survival in a solid tumor, and the first orexin agonist addressed the root cause of narcolepsy. In metabolic disease, oral versions of blockbuster GLP-1 agonists achieved robust weight loss, while a novel class of aldosterone synthase inhibitors tackled treatment-resistant hypertension by targeting its hormonal driver.
Importantly, longevity biotechnology milestones signaled growing mainstream acceptance. The FDA removed the decades-old black box warning on hormone replacement therapy (HRT), reflecting new evidence of its healthspan benefits. Long-acting preventatives emerged for infectious diseases (a 6-month flu prophylactic and twice-yearly HIV PrEP injection). And critically, after years of preparatory research, the FDA greenlit the first trials of gene-edited pig organ transplants—opening the door to solving organ shortages.
2025’s advances went beyond incremental improvements—they validated entire mechanisms and set new benchmarks. The industry moved from “broad strokes” to high-precision interventions: editing single DNA letters to cure rare diseases, modulating microRNA master-switches to quell inflammation, and re-engineering immune responses to outsmart cancer and autoimmunity.
Notable Breakthroughs
1. A Milestone in Personalized Medicine: The “n=1” CRISPR Cure
Patient: “Baby KJ”
Indication: CPS1 Deficiency
Modality: In Vivo Base Editing
Perhaps the most dramatic demonstration of 2025’s precision medicine ethos was the case of baby KJ—an infant born with a fatal metabolic disorder who became the first patient treated with a personalized gene therapy.1
The Clinical Challenge:
KJ had severe carbamoyl phosphate synthetase 1 (CPS1) deficiency, a urea-cycle enzyme defect that caused toxic ammonia to build up to >1000 μmol/L (over 30× normal). Newborns with this condition face imminent brain damage or death, and the usual remedy (liver transplant) is often too dangerous in a neonate.
The Custom Solution: In just seven months from diagnosis, the team designed an adenine base editor specifically targeting KJ’s CPS1 mutation. This gene-editing tool changes a single DNA letter (A→G) without cutting the DNA strands, thereby correcting the disease-causing mutation with minimized off-target damage. The editing mRNA and guide RNA were delivered via liver-targeted lipid nanoparticles (LNPs)—no viruses needed.
Outcomes:
The results were remarkable. The treatment was well-tolerated with no serious side effects. Within weeks, KJ’s ammonia metabolism improved—he could tolerate more dietary protein with his nitrogen-scavenger medications cut in half. Crucially, typical childhood infections that would normally trigger lethal ammonia spikes no longer did so. By mid-2025, KJ was discharged home and was “thriving,” meeting normal developmental milestones that would have been unthinkable for this disease. This single-patient success definitively proved that in vivo base editing can safely and effectively cure a deadly genetic disease.
2. The “GenAI-First”: New Medicine Discovered Using Generative AI Showed Promise in the Clinic
Asset: Rentosertib (ISM001-055)
Company: Insilico Medicine
Indication: Idiopathic Pulmonary Fibrosis (IPF)
If 2024 was the year of AI hype, 2025 was the year of AI proof. For the first time, a molecule designed entirely by generative AI algorithms achieved clinical validation, shifting the narrative from computational potential to patient outcomes.
The Breakthrough: Rentosertib targets TNIK (Traf2- and Nck-interacting kinase), a novel fibrosis target identified by AI, with a molecular structure also designed by AI. In Phase 2a trials, the drug met its primary safety endpoints. However, the efficacy data garnered the most attention: patients receiving the 60 mg daily dose showed a +98.4 mL improvement in Forced Vital Capacity (FVC) over 12 weeks, compared to a decline in the placebo group.
Clinical Context: While the efficacy signal is a promising trend, it is important to note that the trial is early, and confirmatory studies are required to establish long-term benefit. Nonetheless, this result serves as the first proof-of-concept that “silicon-first” discovery can yield molecules that engage novel biology and produce functional improvements in complex human disease.
3. Nobel Science Hits the Clinic: Treg Immune Modulation
Asset: Rezpegaldesleukin (REZPEG)
Company: Nektar Therapeutics
Mechanism: IL-2 Receptor Agonist (Treg Selective)
In 2025, the Nobel Prize in Medicine recognized the discovery of regulatory T cells (Tregs)—the immune system’s brake pedals. Fittingly, this same year saw clinical triumph for a drug that activates Tregs to treat autoimmune disease.
The Data: Rezpegaldesleukin is an engineered IL-2 cytokine that selectively expands Tregs. In a Phase 2b trial for severe eczema (atopic dermatitis), it showed a 61% improvement in skin severity (EASI) at the high dose vs. 31% for placebo. This was achieved by boosting patients’ own Treg cells rather than broadly suppressing immunity. The results—a rapid onset and dose-dependent efficacy—signal a potential paradigm shift in treating autoimmunity by restoring immune balance instead of using blanket immunosuppression.
4. Epigenetic Control: microRNA Modulation
Asset: Obefazimod
Company: Abivax
Mechanism: miR-124 Upregulation
A first-of-its-kind microRNA-targeted drug succeeded in Phase 3 in 2025. Obefazimod is an oral small molecule that boosts the production of miR-124, a natural “master brake” on inflammation.
The Mechanism: The increased miR-124 simultaneously downregulates multiple cytokines like IL-6, IL-17, and TNF. In ulcerative colitis, obefazimod’s Phase 3 induction trials met all endpoints: significantly higher remission rates at 8 weeks compared to placebo (e.g., 25 mg dose yielded ~21% absolute placebo-adjusted remission). This validates miRNA upregulation as a scalable therapeutic strategy—essentially an upstream approach to broadly quell inflammatory pathways via a single “switch.”
5. Regenerative Medicine: Exosome Therapy
Asset: Deramiocel (CAP-1002)
Company: Capricor Therapeutics
Indication: Duchenne Muscular Dystrophy (DMD)
Exosomes—nanoscale vesicles secreted by cells—moved from theory to therapy in 2025. Capricor Therapeutics delivered convincing Phase 3 evidence that an exosome-rich therapy can slow the course of Duchenne muscular dystrophy.
Pivotal Results: In the HOPE-3 trial, intravenous deramiocel slowed the decline in upper limb function by ~54% vs. placebo over one year, and slowed cardiac deterioration by ~91% vs. placebo. Specifically, 51.4% of treated patients needed no additional arm surgery (vs. 20.3% placebo). These differences translate into preserving independence and preventing heart failure, validating exosomes as a new class of “drug factories” for regenerative medicine.
6. Infectious Disease Renaissance: New Gonorrhea Antibiotic
Asset: Gepotidacin
Company: GSK
Class: Triazaacenaphthylene Antibiotic
For the first time in over 30 years, a new oral antibiotic class was approved for drug-resistant gonorrhea. Gepotidacin inhibits bacterial DNA replication by a novel mechanism (binding a distinct site on DNA gyrase).
Clinical Impact: In Phase 3, gepotidacin cured ~98% of uncomplicated Neisseria gonorrhoeae infections, including strains resistant to all current therapies. This is game-changing, as gonorrhea had become increasingly untreatable, leaving clinicians with few options beyond older injectables.
7. Malaria Eradication: 99% Cure Rates
Asset: Ganaplacide + Lumefantrine
Company: Novartis
Malaria saw its biggest therapeutic advance in decades with the GanLum regimen. Unlike artemisinin-based treatments (which face rising resistance), this combination uses a new class of non-artemisinin agent.
The Data: In a Phase 3 trial across 12 African countries, the combination achieved 97–99.2% cure rates at 28 days, remaining effective even against artemisinin-resistant strains. Additionally, scientists unveiled a bed net strategy using endochin-like quinolone (ELQ) compounds that kill parasites inside the mosquito, blocking transmission at the source.
8. The “Chemical Vaccine” Paradigm
Assets: CD388 (Cidara) & Lenacapavir (Gilead)
Concept: Long-Acting Prophylaxis
2025 blurred the line between drugs and vaccines by advancing long-acting prophylactics that provide “passive immunity.”
Universal Flu Shield: CD388, a drug-Fc conjugate, links an antiviral to an antibody tail. In Phase 2b, a single injection provided 76% protection against symptomatic influenza for the entire season (6 months), regardless of strain.
Twice-Yearly HIV PrEP: The FDA approved lenacapavir for PrEP. In landmark trials, it showed 100% efficacy (zero infections) with just two injections per year, resolving the adherence issues of daily pills.
9. New Vectors: Gorilla Adenovirus
Asset: Papzimeos
Company: Precigen
Indication: Recurrent Respiratory Papillomatosis (RRP)
Solving the “delivery problem” remains a priority. In 2025, Papzimeos became the first approved therapy using a gorilla adenovirus vector. These vectors are less immunogenic than human viruses and have a larger cargo capacity.
Outcome:
In RRP patients, 51.4% achieved complete disease remission (no surgery for ≥1 year), compared to 0% historically. This “plug-and-play” vector platform could enable future gene therapies that require delivering larger or multiple genes.
10. In Vivo Base Editing: AATD and the Liver
Asset: BEAM-302
Company: Beam Therapeutics
Beyond “n=1” cases, base editing showed promise for broader populations. Beam Therapeutics reported encouraging first-in-human data for Alpha-1 Antitrypsin Deficiency (AATD).
The Data:
In Phase 1/2, a single dose raised functional AAT protein to 12.4 μM (above the protective threshold) and reduced toxic mutant protein by 79%. This suggests the therapy is fixing the genetic defect at the source—producing normal protein and clearing toxic aggregates—validating the safety of editing genes inside the patient’s liver.
11. Cardiovascular Gene Editing: The “One-and-Done”
Asset: VERVE-102
Company: Verve Therapeutics / Eli Lilly
Verve advanced its in vivo base-editing treatment for high cholesterol. VERVE-102 targets the liver gene PCSK9, permanently silencing it to lower LDL.
The Data:
Interim Phase 1b data showed that a single infusion produced robust LDL-C reductions of 53%. Unlike previous attempts, the optimized LNP yielded no significant safety issues. If confirmed, this offers the potential for a “one-and-done” prevention strategy for heart disease, replacing decades of chronic statin use.
12. Bispecific Antibodies in Solid Tumors
Asset: Petosemtamab
Company: Merus
Mechanism: EGFR x LGR5 Bispecific
Historically, bispecific antibodies struggled in solid tumors. Petosemtamab changed that narrative in 2025. It targets EGFR (growth) and LGR5 (cancer stem cells) simultaneously.
Unprecedented Survival: In head & neck cancer, the drug (combined with immunotherapy) delivered a 79% one-year survival rate, with median overall survival not reached. This compares to historical survival rates of ~50%, leading analysts to term the results “unprecedented” and triggering Genmab’s acquisition of Merus.
13. Metabolic Medicine: The Oral GLP-1 Revolution
Asset: Orforglipron
Company: Eli Lilly
While injectables defined the early 2020s, 2025 ushered in the “Oral Era.” Orforglipron is a small molecule GLP-1 agonist that can be manufactured at scale, bypassing the complex supply chains of biologics.
The Data: In Phase 3 trials, it achieved substantial weight loss (~16 lbs or 7.9% in diabetes patients) and successfully maintained weight loss in patients switching from injectables. This asset promises to democratize access to obesity treatment globally.
14. Precision Cardiology: Targeting Aldosterone
Asset: Baxdrostat
Company: AstraZeneca / CinCor
Hypertension is a leading global killer, often driven by the hormone aldosterone. Previous drugs failed because they also blocked cortisol, causing severe side effects.
The Breakthrough: Baxdrostat is a highly selective aldosterone synthase inhibitor. In Phase 3, it significantly reduced blood pressure in treatment-resistant patients without affecting cortisol. This offers a new tool for the hardest-to-treat cardiovascular patients, addressing a hormonal driver of disease that was previously untouchable.
15. Xenotransplantation: Solving the Organ Shortage
Company: eGenesis / United Therapeutics
Milestone: First Formal Clinical Trials
Finally, 2025 marked the transition of xenotransplantation from experiment to clinical trial. eGenesis and United Therapeutics launched first-in-human trials of gene-edited pig kidneys.
The Science: eGenesis’s pigs have 69 genomic edits to remove rejection antigens and viral risks. In a compassionate use case, a patient survived for 8 months with a pig kidney, proving long-term function is possible. The FDA’s green light for formal trials represents a turning point where decades of research have coalesced into a feasible solution to save lives.
16. Quantum Computing’s “Hello World” in Drug Discovery
Assets: ISM061-018-2 & ISM061-22 Source: Nature Biotechnology (Jan 2025) Target: KRAS (Cryptic Pockets)
While AI is optimizing known chemical spaces, quantum computing is beginning to explore the unknown. In January 2025, a landmark study published in Nature Biotechnology provided the first experimental proof that quantum generative models can design valid pharmacological hits.
The Hybrid Approach: Researchers from Insilico Medicine and the University of Toronto utilized a hybrid quantum-classical workflow on a 16-qubit IBM quantum computer. They combined Quantum Circuit Born Machines (QCBMs)—which exploit quantum superposition to efficiently sample complex molecular distributions—with classical Long Short-Term Memory (LSTM) networks to navigate the vast chemical space of KRAS inhibitors.
The Hits: Unlike fully classical models, this hybrid approach synthesized 15 molecules, two of which were confirmed as valid biological hits:
ISM061-018-2: A broad-spectrum inhibitor with a binding affinity of 1.4 µM against the KRAS-G12D mutation.
ISM061-22: A selective inhibitor targeting the G12R and Q61H mutants.
The Significance: While the affinity (micromolar) represents an early “hit” rather than a clinical lead (often nanomolar), the study is foundational. It moves quantum drug discovery from theoretical physics to wet-lab reality, proving that quantum algorithms can generate novel, synthesizable structures that engage difficult biological targets.
17. Airport Gyms: RFK Doing 20 Pull Ups in the Airport
Milestone: Pull-up Bars in Airports
While not a biomedical advance, my longevity highlight of 2025 was the 72-year old Secretary of Health and Human Services doing 20 pull-ups in a suit at the airport in a viral video. Whenever I am at the gym doing pull-ups, I replay his video in my mind and it helps me push a bit harder - if a 72-year old director of HHS can do it, I should be able to do it too. You may or may not like his policies but this Maintaining healthy muscle is among the most important lifestyle modifications anyone can do.
Toward a Longer, Healthier Future
By the numbers, 2025 saw 55 new FDA drug approvals (37 small molecules, 18 biologics)—consistent with recent years’ productivity. But beyond quantity, the year will be remembered for qualitative leaps forward. The overarching theme was precision—therapies honed to specific molecular targets or root causes.
In the realm of longevity biotechnology, 2025 was also notable. Clinical studies provided the first solid evidence that certain interventions can extend healthspan in humans. For example, the PEARL trial showed weekly low-dose rapamycin was safe in older adults. A major regulatory shift saw the FDA remove the Black Box warning from HRT, reflecting growing institutional acceptance of longevity-focused treatments.
In summary, 2025 will be remembered as a year when therapeutic precision and bold scientific bets began paying off for patients. Diseases long thought incurable—from genetic syndromes in infants to degenerative illnesses of aging—were altered fundamentally by new treatments. By attacking root causes and enabling regeneration, 2025’s breakthroughs laid the groundwork for people not only to live longer, but to live healthier, more vital lives.
Disclaimer: This article is written with the help of generative tools so beware of hallucinations. Don’t buy, sell, or take any drugs based on this article or any of its contents. The information and views expressed in this article are for informational and educational purposes only and do not constitute medical advice. The content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or another qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read in this article. The author is sharing personal experiences and opinions. These experiences are not a recommendation or endorsement for any specific treatment, drug, or course of action. The medications and therapeutic strategies discussed may not be suitable for everyone and can have significant risks and side effects. Some of the drugs mentioned are investigational and have not been approved by the FDA or other regulatory agencies for the uses discussed. While the author is the CEO of Insilico Medicine, the statements and view presented in Forver.ai do not represent the views and opinions of Insilico Medicine.